PGD/PGS: Old Technology Preimplantation Genetic Diagnosis - The Old Technology
Published: 12/11/2015
First developed in 1968, preimplantation genetic testing is a technique used to identify a chromosome or genetic abnormality in embryos created using In-Vitro Fertilization (IVF) before transferring those embryos into the uterus. Preimplantation Genetic Diagnosis (PGD) specifically refers to when one or both genetic parents are carriers for a known genetic/chromosome abnormality, and testing is performed on an embryo to see if it also carries the same abnormality.
In contrast, Preimplantation Genetic Screening (PGS) refers to techniques where embryos from presumed chromosomally normal genetic parents are screened for spontaneous chromosome abnormalities or a Euploidy. A Euploidy is an abnormal number of chromosomes, consisting of an extra (trisomy) or missing chromosome (monosomy) (a common cause of genetic disorders (birth defects)). It is called PG screening rather than PG diagnosis because of mosaicism (or two different cell lines in an embryo, where one could be normal and the other abnormal (the biopsy could randomly get the one normal cell where the rest are abnormal, or vice versa)), or lack of 100% accuracy (because of overlaying cell signals using FISH (see below)). PGS is only 90% accurate in identifying chromosome abnormalities.
Chromosome abnormalities occur in approximately 1 of 160 live births, the most common being extra chromosomes in the 21 (Down's Syndrome or Trisomy 21), 18 (Edward's Syndrome or Trisomy 18) and 13 positions. Aneuploidy occurs very early during embryo cell division when the chromosomes do not separate properly between the two cells.
Today, the term “PGD” is used to describe all types of preimplantation genetic testing. Since only chromosome normal embryos are transferred into the uterus, PGD provides an alternative to current post-conception diagnostic procedures (like amniocentesis or chorionic villus sampling (CVS)), which are associated with certain risks and complications. If abnormal, the couple is faced with the difficult decision of whether or not to terminate the pregnancy. Both amniocentesis and CVS procedures are associated with a 1 in 200 to 1 in 250 risks of serious complications resulting in pregnancy loss.
Preimplantation Genetic Diagnosis is currently the only option to screen embryos before implantation for chromosome abnormalities carried by the genetic parents. Any woman who is at risk of a chromosome abnormality because of her age or history (i.e., repeated pregnancy loss) who does not want to go through the physical, emotional, and psychological trauma of a miscarriage (60-70% of all miscarriages are due to spontaneous chromosome abnormality), or is interested in gender selection can do this prior to embryo transfer.
PGD is also used for diagnosing and preventing single-gene mutations, which are certain inheritable diseases resulting from a single abnormal gene sequence, or X-linked disorders which are disorders carried on the sex chromosome, thereby eliminating the difficult decision of pregnancy termination.
Preimplantation Genetic Diagnosis (PGD) Indications
Arizona Center for Fertility Studies offers PGD for the following indications:
- Sex-linked or X-linked disorders
- Single gene mutations or defects
- Chromosome abnormalities
Sex-Linked or X-Linked Disorders
X-linked diseases are passed by an abnormal X chromosome to the child through a carrier mother. They manifest in sons, who do not inherit the normal X chromosome from the father (sperm are either X or Y). Because the X chromosome is transmitted to the embryos through the mother, affected fathers with abnormal X but normal Y chromosomes have sons who are not affected, but their daughters have a 50% risk of being carriers if the mother is healthy. Sex-linked recessive disorders include hemophilia, fragile X syndrome, most neuromuscular dystrophies, and hundreds of other diseases.
Single Gene Mutations or Defects
Preimplantation Genetic Diagnosis is used to identify single-gene defects, such as cystic fibrosis, sickle cell anemia, Huntington Chorea, Tay-Sachs, and many others. In these diseases, the abnormality is detectable by placing a single cell from the embryo (blastomere) in a buffered solution and sending it for molecular techniques using Polymerase Chain Reaction (PCR) amplification of DNA from a single cell.
PCR is the process used to amplify the genetic sequence in question to obtain a sufficient quantity of the gene portion to be analyzed for the specific defect. Once embryos are developed, a blastomere is withdrawn from each embryo and tested by PCR for the specific single gene mutation to determine if each embryo will be affected by the disorder, be a carrier, or not affected at all. PGD can also be used to identify genetic mutations like BRCA-1, which statistically appear to increase a woman’s risk of breast cancer.
Chromosome Abnormalities
The last category using PGD includes chromosomal disorders resulting from a carrier state of one or both of the parents, in which a variety of chromosomal rearrangements (including translocations, inversions, and deletions) can be detected using Fluorescent In-Situ Hybridization (FISH).
Chromosomal translocation is a condition where part of a chromosome has broken off and reattached in another location. Translocations can be completely harmless, or they can cause serious health problems depending on the circumstances.
In the case of the former, many people can have translocations without being aware. This is usually the case for reciprocal (or balanced) translocation, a type of chromosomal translocation that increases the risk of recurrent miscarriages. In a balanced translocation, a person usually has all the genetic material necessary for normal growth. However, when that person's cells divide to create egg or sperm cells, these cells can end up with extra or missing genetic material, which could lead to miscarriage depending on which chromosome and genes are affected. In about 4.5% of all couples with repeated pregnancy loss, one or both parents has a balanced translocation.
An inversion is a chromosome rearrangement in which a chromosome segment is reversed end to end. An inversion occurs when a single chromosome undergoes breakage and rearrangement within itself.
FISH is used to detect the number of a set of chromosomes (or pairs of chromosomes) present inside the blastomeres, or a specific loci site of interest on the chromosome.
The nucleus must be isolated to determine the numerical chromosome content of the cell or specific loci (a process called fixation). The cell is placed on a glass slide and treated with chemicals to wash away the cytoplasm containing the cellular machinery while affixing the nucleus containing the chromosomes onto the slide. Probes for the desired panel of chromosomes or sites are allowed to bind to the chromosomes on the glass slide (a probe is a small molecule designed to bind to a specific chromosome or specific site on the chromosome, and fluoresce or "glow" when exposed to UV light). The fluorescent results are used to tally the number of chromosome pairs present inside the cell.
Each of a cell's 23 chromosomes is made up of 2 pairs; one from the mother and one from the father. One of the drawbacks of FISH that occasionally occurs is that two signals (one from each pair) from a given chromosome will overlap, giving off only one signal rather than two, making it look like that chromosome only has one pair, or be monosomy.
The "future" parents are carriers for these chromosome rearrangements that are not lethal to them (or "balanced"), but can cause unbalanced chromosome abnormalities in their embryos that are incompatible with life, resulting in the inability to produce a normal embryo that can implant, or that will implant only to end in an early miscarriage. Some couples may never achieve a successful pregnancy and/or will experience repeated pregnancy loss because one or both of them are carriers for undiagnosed non-lethal chromosome rearrangements.
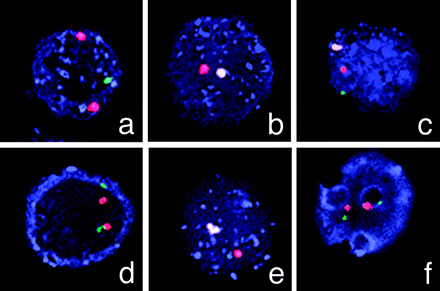
An example of using FISH to screen embryos for neuromuscular disorders; in this case, muscular dystrophy. Hybridizations on single blastomeres (cells) from 6 out of 8 embryos during the first Preimplantation Genetic Diagnosis (PGD) cycle of the Duchenne muscular dystrophy patient show a normal male embryo (c), two normal female embryos (d and f), a carrier female embryo (a), and two affected male embryos (b and e). Courtesy of Basic Science of Reproductive Medicine, volume 12, number 5, pages 353-356.
Preimplantation Genetic Screening (PGS) Indications
Arizona Center for Fertility Studies also offers Preimplantation Genetic Screening (PGS) (commonly confused and typically called Preimplantation Genetic Diagnosis (PGD)) for Aneuploidy, and screening embryos of women that are at increased risk of spontaneous chromosome abnormalities (most commonly, a trisomy or monosomy).
Arizona Center for Fertility Studies offers PGS for the following indications:
- Women 38 or older
- Couples with repeated pregnancy loss
- Women with repeated IVF failure
- Sex or gender selection
Aneuploidy increases with a woman's age and is not determined by "how good of shape she is in or how good she looks;" it is strictly related to the woman's age and reproductive genetics. Was she blessed with good reproductive genes, or not so good reproductive genes? Unfortunately, there is no way of telling this in advance; although, having a family history on the maternal side of women delivering healthy normal babies late in reproductive life is a good sign.
As a woman ages, her eggs age with her. Every month that she ovulates one egg, she loses a 1,000. The egg that becomes dominant and ovulates in a given month is random and not determined by the "better eggs being released first." The remaining eggs then age as the woman ages, putting them at increased risks of spontaneous chromosome damage.
Most of the time, a chromosomally abnormal egg will not even fertilize, and if it does, it will not implant. If it does implant, it will almost always result in a miscarriage. Occasionally, one can "slip through" and result in a live birth, the most common being Trisomy 21 or Down's Syndrome. Rarely, a Trisomy 18 or Edward's Syndrome embryo will result in a live birth, but unlike Down's, it will always die within the first two to three months of birth.
As a woman ages, the chromosomes of the remaining eggs are less likely to divide properly, leading to extra or missing chromosomes, or aneuploidy. The rate of aneuploidy is greater than 20% in mothers aged 35-39 years and is nearly 40% in mothers aged 40 years or older, leading to the increased rates of miscarriages in reproductively older woman. The rate of aneuploidy resulting in live births (e.g., Down's or Edward's Syndrome) is 0.6-1.4% in mothers aged 35-39 years and 1.6-10% in mothers older than 40 years. The difference in percentages between affected embryos and live births is because an embryo with aneuploidy is less likely to be carried to term and will most likely be miscarried, some even before pregnancy is suspected or confirmed.
Arizona Center for Fertility Studies believes that using PGD/PGS to determine the chromosomal composition of embryos prior to embryo transfer increases the chance of a healthy pregnancy and reduces the risk of miscarriages and babies born with chromosome abnormalities.
The following chart shows the incidence of Down's Syndrome, or Trisomy 21, diagnosed at 16 weeks by amniocentesis or CVS versus the incidence of Down's Syndrome babies at birth; suggesting that although most Down's embryos will not implant or result in an early miscarriage, there is the possibility for a Trisomy 21 to result in a live birth.
Although some couples will choose to terminate a Down's pregnancy when they find out, others will not, and go on to deliver the pregnancy. Down's children are lovable and can live for several decades; however, they are a life-long commitment that affects the entire family unit. PGD is the only option available to not have to face this extremely difficult and very personal decision.
| Age | Incidence of Down's Syndrome detected at 16 weeks | Incidence of Down's Syndrome at birth |
|---|---|---|
| 15-19 | ... | 1/1250 |
| 20-24 | ... | 1/1400 |
| 25-29 | ... | 1/1100 |
| 30-31 | ... | 1/900 |
| 32 | ... | 1/750 |
| 33 | 1/420 | 1/625 |
| 34 | 1/325 | 1/500 |
| 35 | 1/250 | 1/350 |
| 36 | 1/200 | 1/275 |
| 37 | 1/150 | 1/225 |
| 38 | 1/120 | 1/175 |
| 39 | 1/100 | 1/140 |
| 40 | 1/75 | 1/100 |
| 41 | 1/60 | 1/85 |
| 42 | 1/45 | 1/65 |
| 43 | 1/35 | 1/50 |
| 44 | 1/30 | 1/40 |
Screening for Aneuploidy
Arizona Center for Fertility Studies performs PGS for aneuploidy screening using the most current next-generation sequencing technology, which is highly reliable for the detection of partial and complete chromosome abnormalities involving all 23 chromosome pairs.
For more information of PGD testing, please call any of Arizona Center for Fertility Studies’ medical team, or call us at (480) 860-4792.
Preimplantation Genetic Diagnosis and Gender Selection
Finally, and although never intended for this use, a popular use of PDG is for gender selection (to determine the sex of the embryo) for "family balancing," or for social, psychological, or cultural reasons.
There is an ongoing controversy about using the PGD technology for determining the sex of the embryos. Not that there is necessarily an argument against family balancing, "wanting a boy rather than a girl, or vice versa," it is more of what is done with the embryos of the undesired sex?
If these "unwanted" embryos are donated anonymously to another couple, that is one thing; but many times they are discarded, raising all kinds of moral, ethical, religious, spiritual, and psychological issues for the parents, the clinic, their staff, and society as a whole.
Arizona Center for Fertility Studies respects a couple's right to choose, and although encourages the anonymous donation of "extra embryos," will honor the couple's choice to do PGD for sex selection and their decision on the disposition of the extra embryos.
In order to do PGD or PGS, the couple needs to initially do In-Vitro Fertilization (IVF). If there is a risk or known risk of chromosome rearrangements or single-gene mutations, or history of repeated pregnancy loss, then the proper genetic and psychological counseling will be done before starting the process.
IVF is then done in the standard way, along with Intracytoplasmic Sperm Injection (ICSI) to increase fertilization rates and the number of embryos obtained; as well as assure that only one sperm enters the egg, thus not adding any extra chromosomes (e.g., 2 sperm getting into the egg) that will be picked up by PGD. The embryos are cultured to day 3, then a single cell (blastomere) is removed from each embryo, and the nucleus of that cell is fixed on a slide and sent for evaluation of the chromosomes. This is done for all of the embryos obtained on that cycle.
Embryo biopsy, or the removal of a blastomere, is difficult, but can be done safely and without any damage to the embryo in the hands of an experienced embryologist. To date, with hundreds of PGD procedures performed by the Arizona Center for Fertility Studies’ embryology laboratory, not a single embryo has been damaged.
Only a blastomere is removed from each embryo. Although removing 2 blastomeres would give almost a 100% accurate diagnosis of each embryo’s chromosome makeup, data shows that there are decreased pregnancy rates when 25% or more of the mass of the embryo is removed. Normal day 3 embryos are 7, 8, or 9 cells, and by only removing one cell (or around 12-13% of the mass of the embryo), it does not appear to affect pregnancy rates and outcome. The trade-off is that with only evaluating one cell of an embryo, PGD is about 90% accurate in diagnosing a chromosome abnormality. Said differently, there is a 10% chance that PDG will "miss" a chromosome abnormality, or report an embryo to be abnormal that is actually normal. Arizona Center for Fertility Studies thinking is, "that it is better to know 90% of the time the status of an embryo at risk than none of the time."
Usually, the results of PGD are done in 24 hours, and once the chromosome complement of each embryo is known, embryo transfer can be done on day 4 (morula) or day 5 (blastocyst).
Additional methods of doing PGD are to biopsy the polar body, or small round mass extruded from the embryo at the time of ovulation that contains a single set of 23 chromosomes (the egg prior to ovulation has 46), making room for the single set of 23 chromosomes coming in from the sperm. Polar body biopsy only works to evaluate female chromosomal disorders. PGD can also be done on a blastocyst or day 5 embryo by doing a biopsy of the outer ring of cells of the embryo, which will eventually form the placenta (known as the trophectoderm). The risk of doing this type of biopsy is getting cells that are not representative of the embryo. Arizona Center for Fertility Studies does all PGD biopsies on a day 3 embryo that is, on average, 6-9 cells.
A blastomere biopsy is done by holding the embryo at 9 o'clock with a micro-holding pipette; simultaneously, a micro-manipulation pipette containing acidic tyrode solution is placed adjacent to the zona pellucida, or wall of the embryo. The tyrode solution is gently extruded to thin the zona and slowly make a small opening. The manipulation pipette is placed through the opening in the zona, and a single blastomere is carefully removed and gently aspirated into the pipette and expelled into the surrounding medium. After all the embryos have undergone biopsy and placed in their individually numbered dishes corresponding to the embryo that they were removed from, the cytoplasm is washed away and the nucleus (containing the chromosomes) is fixed to a slide to be sent for evaluation. The embryos are then placed back in the incubator while awaiting the results.
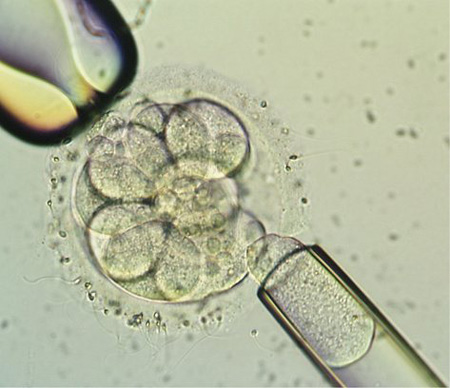
Picture of an 8+ cell embryo undergoing embryo biopsy, with a micro-holding pipette on the left and a micro-manipulating pipette on the right gently removing a single cell or blastomere for Preimplantation Genetic Diagnosis (PGD) analysis.
Video made at Arizona Center for Fertility Studies of a normal embryo undergoing embryo biopsy for Preimplantation Genetic Diagnosis (PGD).
Even after a successful pregnancy, it is still recommended by some Obstetricians that either amniocentesis or CVS be done since PGD is not 100% accurate in diagnosing chromosome abnormalities (about 90%). Arizona Center for Fertility Studies does not recommend this, but the couple is already aware of this possibility and the decision is up to them. Most do not choose to do an amnio or CVS since the risks of having a complication from the procedure is much higher than the odds of "missing" a chromosome abnormality. To date, there are thousands of babies born who underwent PGD as embryos with no reported statistical increase in birth defects or other identifiable problems.











